

Biopharmaceutical / Biotechnology Innovator.






Leveraging the Power of AI.


Building Global Partnerships.

INNOVATIVE DIAGNOSTIC AND THERAPEUTIC APPLICATIONS
Biopharmaceutical / Biotechnology Innovator
We are a disruptive emerging biopharmaceutical/biotechnology innovator, challenging the status quo by shifting traditional medical paradigms. We are pushing boundaries and driving innovation by turning groundbreaking science into cutting-edge diagnostics and therapeutics.



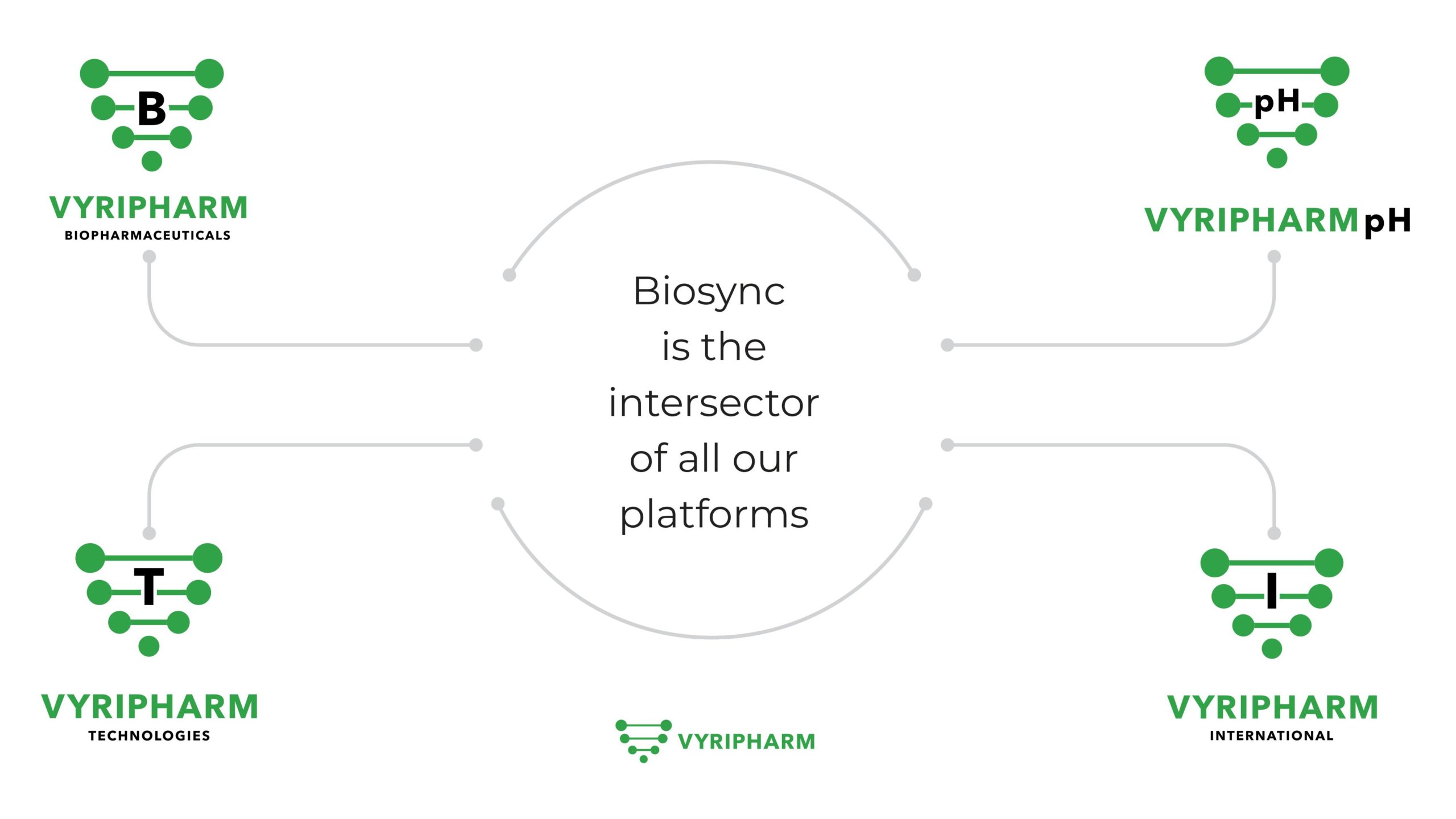
BIOSYNC TECHNOLOGY
Our BioSync™ Platform
The BioSyncTM Platform has been designed as the first and only platform that integrates agribiopharmaceuticals with biopharmaceuticals and biotechnology.
This advanced data procurement creates a world-class, robust, and innovative data management platform.

ALTERNATIVE MEDICINE
Theranostics combines diagnostics and therapeutics providing a transition from traditional medicine to personalized, patient-centered medicine.
Research Initiatives
Hemp/Cannabis Testing Standard and Genetic Classification: A Framework for Innovation, Regulation, and Economic Growth.
Please fill out the form below and press "Submit" to access the white paper.
*All contact details will be kept confidential.
Vyripharm Enterprises, Inc. and Colorado State University-Pueblo Announce Sponsored Research and Facilities Agreement.
Vyripharm International, Inc. and the National Yang-Ming University Signed a MOU to Develop VYR-206 as a Novel Diagnostic Approach for Neurological Disorders Outcomes Following Treatments.
Vyripharm biopharmaceuticals and Regis Technologies, Inc. Have Signed Off on a Master Drug Program Agreement for Expediting cGMP Manufacture of Our Antiviral Agents for Diagnostic Evaluation of COVID-19.
Vyripharm biopharmaceuticals Is in the Development Stage of a Novel Integrated Theranostic Approach for the Treatment of Viral Infections Such as COVID-19.

ALTERNATIVE MEDICINE

