yripharm Enterprises Inc and ABB, Inc. collaborate on the design and development of Automation and Robotics to advance Testing Laboratory Platform for Public Health and Public Safety
HOUSTON--(BUSINESS WIRE)--Vyripharm Enterprises Inc. (VEI), a Texas biopharmaceutical/biotechnology company based in the Texas Medical Center (TMC) in Houston, and ABB Robotics, a leading global technology company headquartered in Zürich, Switzerland, have signed an agreement towards the development of an automated laboratory testing platform.
Vyripharm Enterprises Inc. held its 2022 Legislative Summit For Public Health and Public Safety with Keynote Speaker Congressman Dan Crenshaw
HOUSTON--(BUSINESS WIRE)--Vyripharm Enterprises Inc. and BIZPAC.US hosted the First Annual Vyripharm Public Health and Safety Legislative Reception on August 31, 2022 at the Texas Medical Center (TMC) Institute Innovation Factory, TMC's 1st phase biotech incubator and accelerator.
Vyripharm Enterprises, Inc. and Colorado State University Pueblo Announce Sponsored Research and Facilities Agreement
HOUSTON & PUEBLO, Colo., March 10, 2022--(BUSINESS WIRE)--Vyripharm Enterprises, Inc. and CSU Pueblo announced today a partnership to support the creation of a comprehensive testing and certification laboratory that will provide the first consistent scientific analysis within cannabinoid research.
Increased Oversight of the Cannabinoid Industry Reduces the Risk to Public Health and Public Safety.
Since 2012, marijuana has become legal for adult consumption in 16 states and the territory of Washington D.C. Medical marijuana is legal in 36 states at the time that this summary was written. There are currently three other states that have either voted or passed laws that have not gone into effect concerning marijuana consumption.
Vyripharm International, Inc. and the National Yang-Ming University Signed a MOU to Develop VYR-206 as a Novel Diagnostic Approach for Neurological Disorders Outcomes Following Treatments.
HOUSTON–(BUSINESS WIRE)–Vyripharm International, Inc. (VI), is a subsidiary of Vyripharm Enterprises, LLC, a privately held company formed for Partnerships and Joint Ventures with International Public or Private Organizations.
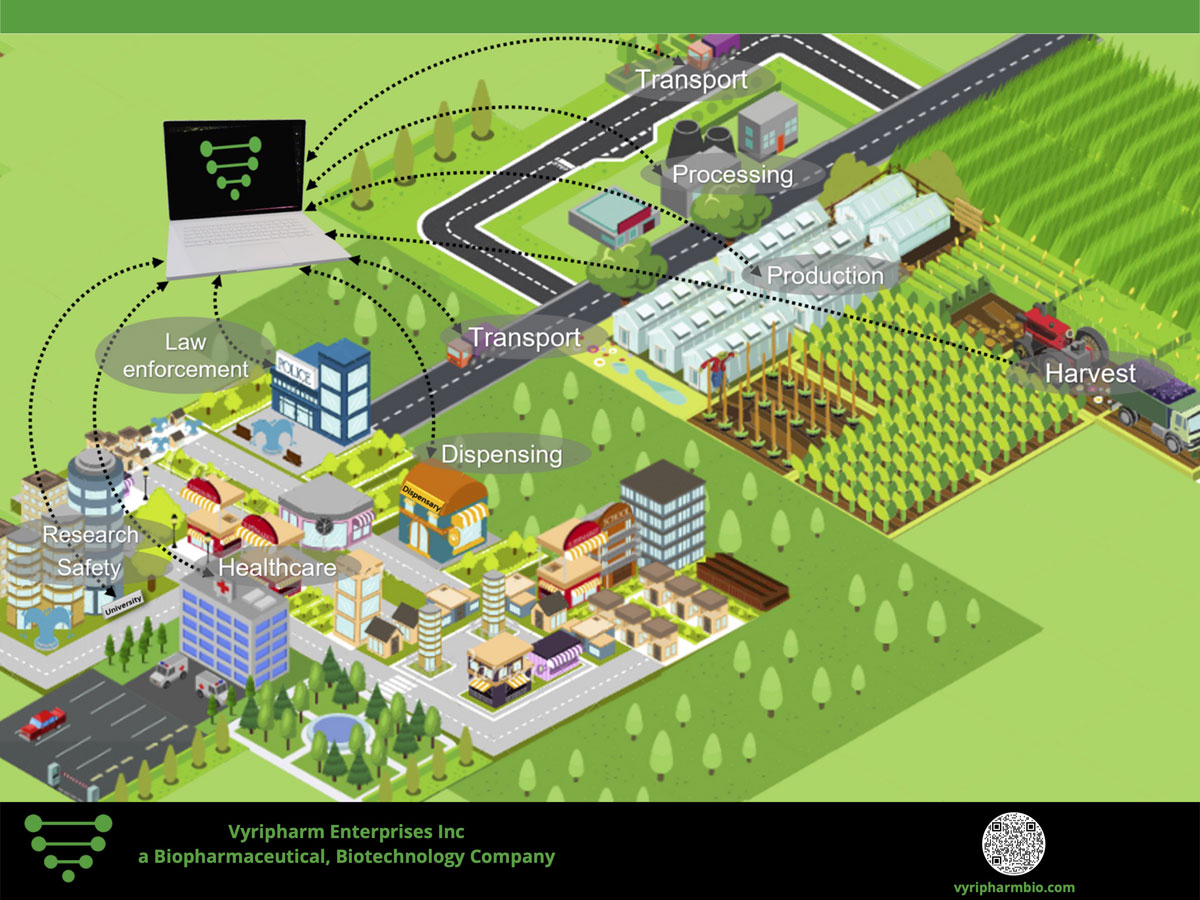
Vyripharm Biopharmaceuticals Issued Patent for Comprehensive Medical Cannabis Testing from Agriculture to Clinical Application
Raising the Bar for Comprehensive Medical Cannabis and Hemp Testing and Evaluation Vyripharm biopharmaceuticals, a privately held biopharmaceutical company located in the Texas Medical Center in Houston, Texas is pleased to announce that it has been granted patent allowance by the United States Patent and Trade Office for its comprehensive medical cannabis and hemp testing methodology platform.
Vyripharm Biopharmaceuticals and Regis Technologies, Inc. Have Signed Off on a Master Drug Program Agreement for Expediting cGMP Manufacture of Our Antiviral Agents for Diagnostic Evaluation of COVID-19.
HOUSTON–(BUSINESS WIRE)–Vyripharm biopharmaceuticals, a subsidiary of Vyripharm Enterprises, LLC, a privately held company located in the Texas Medical Center in Houston, Texas, announced today that it has signed off on a Master Drug Program Agreement with Regis Technologies, Inc.
Vyripharm Biopharmaceuticals Is in the Development Stage of a Novel Integrated Theranostic Approach for the Treatment of Viral Infections Such as COVID-19.
HOUSTON–(BUSINESS WIRE)–Vyripharm biopharmaceuticals, a subsidiary of Vyripharm Enterprises, LLC, which is a privately held company located in the Texas Medical Center in Houston Texas, announced today that it will repurpose the development of a novel theranostic platform for the diagnosis, monitoring and treatment of viral infections such as SARS, MERS and COVID-19.